

|
||||
| Home | Services | CDISC | Software | About us |
Download the newest user manual (v.2022)
The define.xml file is now a fixed and mandatory part of any electronic submission of CDISC SDTM, SEND or ADaM datasets to as well the FDA as PMDA regulatory agencies. Still, there are very little tools to generate a high-quality define.xml file.
The Define-XML Designer is the most user-friendly of them. It is the only tool so far on the market that is based on user-friendly "wizards" and where the user has control at all times
of what exactly is produced.
The new version 2022 has full support for as well Define-XML 2.1 as 2.0, and the very old version 1.0.
Competitor tools require you to provide details of the define.xml (after having generated a prototype from the XPT files) using an Excel worksheet. No manual how to do this is however provided. It is suggested to take an existing define.xml and to generate an Excel worksheet from that and edit the latter, adapting it for your own study, and then generate a new define.xml for your own study. A doom loop indeed! Other tools use an "Excel-like" GUI, but still have the "black box" issue. In the time you have learned how that works (and leading to a result that you do not understand yourself - as it is "black box"), you will already have done the job using the Define-XML Designer, AND obtain a define.xml that you do understand.
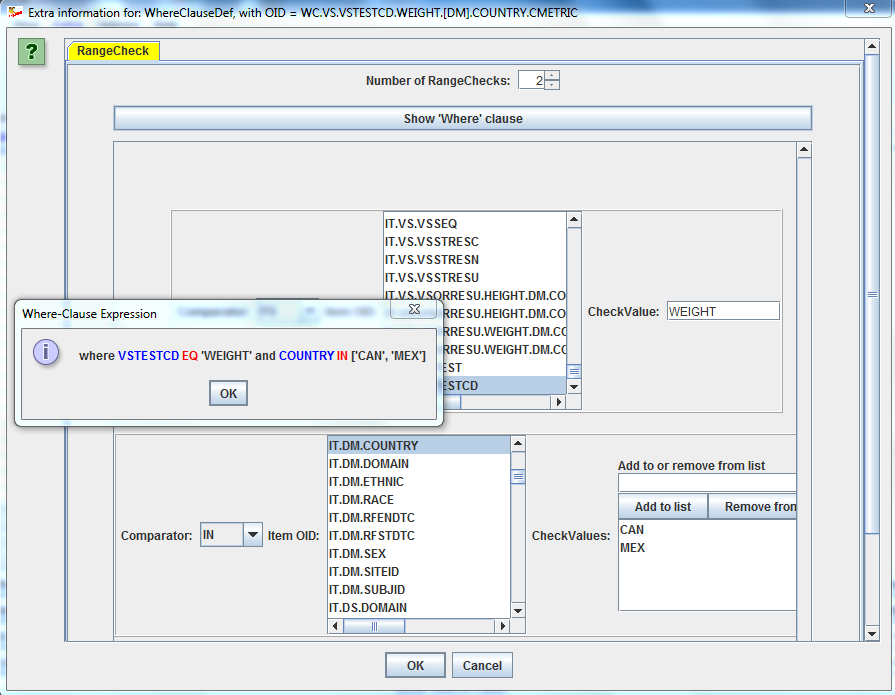
Using Excel (or an Excel-like GUI) for providing the details of the define.xml is extremely user un-friendly. The Define-XML Designer uses Wizards to allow you to specify details of the define.xml, for example to specify links to an external document for your SDTM/SEND/ADaM variable or comment (left picture) or to add all necessary "Where clauses" (right picture) in an extremely user-friendly way:
 |
 |
Want to know more? Then simply download the User Manual (v.2022 - regularly updated and extended). It contains very good explanations of all the features of the software.
When working in the define.xml design mode, you will always be able to inspect what you have generated.
With just two clicks, you will be able to see the generated define.xml in your favority viewer or browser, either using the CDISC stylesheet, or using your own stylesheet.
Even more, you will ALWAYS be able to inspect the underlying XML that is generated in the background (so NOT "black box"), or to obtain a tree view of your study.
Unlike in other tools, validation of your define.xml is done using the rules developed by CDISC Define-XML team (and not the interpretation of the standard by a single vendor). For example, the software validates using the guidelines in the "XML Schema Validation for Define.xml White Paper", published by CDISC.
The Define-XML Designer allows to either start from (included) templates for all newer versions of SDTM, SEND or ADaM, or from a submission-ready set of SAS-XPT files. This allows either to generate the define.xml already before study start (after study design), or near the very end of the prccess, where submission-ready SAS-XPT files are available and all that is still missing is the define.xml.
If your company implements "CDISC end-to-end" yet, and in addition to generate study designs, you want to use the software to set up or generate define.xml files (either from scratch, SDTM/SEND templates or starting from a set of SAS-XPT files), we can offer you the "ODMDesigner.html" full software which already contains the Define-XML Designer as a module. The reason is simply that Define-XML is essentially an extension to ODM.
Even more ideal than needing to design the define.xml "offline" using a tool like the Define-XML Designer, is to have a process in place that creates the mapping between operational data and the SDTM/SEND domains and datasets, and that automatically generates and synchronizes the define.xml in the background. Our SDTM-ETL software is the ideal tool for this as has already been discovered by many sponsors and service providers. Also SDTM-ETL is NOT a black-box tool as one always can inspect the underlying define.xml source. Just like the ODM Study and Define.xml Designer, it uses many Wizards to allow the users to specify the details of the define.xml in a very user-friendy way.
For more information, or if you would like to obtain a copy of the trial version, or obtain pricing information, do not hesitate to send us a mail.
You may also want to have a look at the CDISC ODM Study Designer
