

|
||||
| Home | Services | CDISC | Software | About us |
|
Designed and programmed by one of the main developers of both the ODM and the Define-XML standard! Click here if you are only interested in creating and designing define.xml files More and more, it becomes clear that submissions to regulatory authorities ideally should already be prepared even before the study starts, i.e. at study design time ("upfront"). This principle is known as CDISC end-to-end. Also, define.xml is just an extension of ODM, so why use two different tools for two highly related activities, the study design for starting a clinical study, and preparing a define.xml for a future submission, based on the same information and partially containing the same content? More and more sponsors start using define.xml as a specification for their CRO or service provider, specifying how they want to receive the SDTM/SEND datasets when the study is finalized. Ideally, the SDTM/SEND uses the same controlled terminology as the CRFs and the clinical database, the latter usually being set up using an ODM study design. The controlled terminology itself is regularly published by CDISC as ... ODM. So we decided to publish one tool for both designing studies in ODM as well as for designing define.xml for your FDA and PMDA submissions.  What the ODM Study and Define.xml Designer is NOTIf you would like to automatically generate a define.xml from your SDTM/SEND/ADaM SAS-transport files, WITHOUT understanding what you did (usually leading to a terrible result), the ODM-define.xml Designer is NOT the right tool for you.
There are other such low quality tools on the market, even free ones. Also see the article: Creating define.xml - best and worst practices Advantages for sponsorsUsing the ODM Study and Define.xml Designer, sponsors can use one single tool to either design the complete study as CDISC ODM, use their own libraries with CRFs, sponsor-defined visits, and sponsor and/or
CDISC defined controlled terminology,
AND/OR, develop a define.xml as a specification as how they want to obtain the submission files at study finalization. Advantages for CROs and service providersWhen receiving a study design as CDISC ODM, CROs can use the Designer to further develop the details of the study, such as adding range checks, developing skip conditions, and,
in case not already done by the sponsor, add CDISC controlled terminology (highly recommended) or their own controlled terminology, and this in an extremely user-friendly way. 1. Study Design in ODMWhen using the software in "Study Design" mode (even when starting from a define.xml), one can use all features of the CDISC ODM standard, such as skip questions (or groups of them) and SDTM/SEND annotation (allowing to later make a jumpstart when generating SDTM/SEND files), as well as vendor-specific features of study design, as tailored versions for all major ODM-extensions of EDC vendors are available (e.g. OpenClinica, Clincapture, Medidata, ...). You can also choose to use the CDISC SDM-XML extension, allowing to define detailed workflows, set the study design parameters (to be used later in SDTM), and e.g. to automatically generate a caBIG Patient Study Calendar. More details about the ODM Study Design features  2. Designing and generating define.xmlBesides SDTM-ETL, the ODM Study and Define.xml Designer is currently the best tool on the market for generating Define-XML files. It either allows you to generate a define.xml in a very user friendly way:
Where you always have the choice between define.xml 1.0 and define.xml 2.0. In each of the cases, you can immediately load all necessary controlled terminology (the tool has all the CDISC-CT versions since 2013). In case new controlled terminology is published, you can just add the file with it in a specific directory, and you can then use it immediately (no software update necessary) More details about the Define-XML Design features 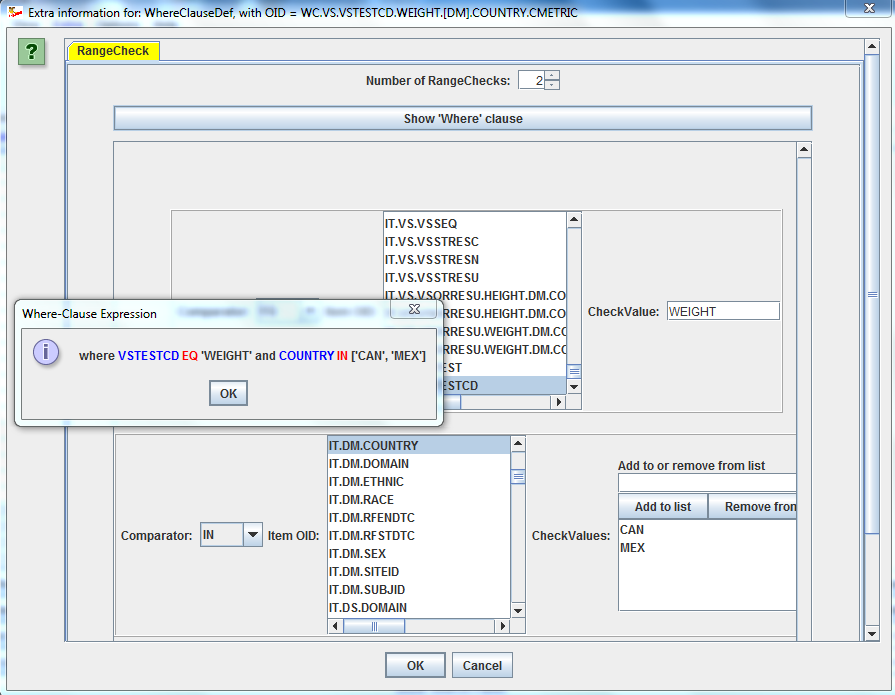 More info?For more information, or if you would like to obtain a copy of the trial version, or obtain pricing information, do not hesitate to send us a mail.

XML4Pharma, Katzelbachweg 18, A-8052 Thal, Austria |