The CDISC Define.xml Checker
Upon request of many of our customers, who are frustrated about the Define-XML validation tools that are currently available, and especially
the one used by some regulatory authorities,
we developed our own Define-XML validation tool, this time implementing rules developed by the CDISC Define-XML Team (and not by
programmers that have never been involved in the development of the standard),
and based on the CDISC XML Schema Validation for Define.xml White Paper
and Schematron technology
As one of the developers of the Define-XML standard, and as a CDISC Authorized Define-XML Instructor,
we know every detail of the Define-XML standard extremely well, and thus are the first choice for a Define-XML validation tool.
The Define.xml is not a free tool, but for that, you get real quality, unlike other tools that are free, but mix up the rules for define.xml v.1.0 and v.2.0, and generate many false-positive errors.
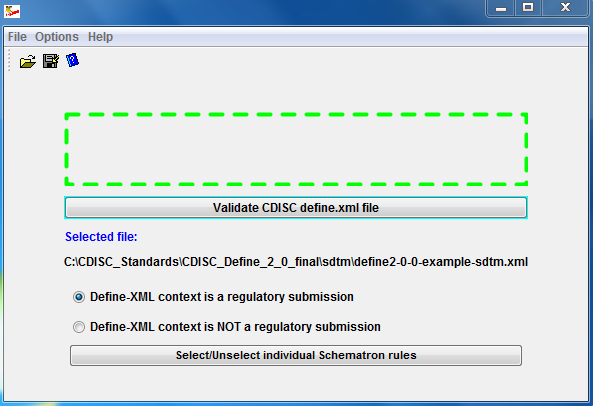
Instead, the Define.xml Checker provides:
- Automatic recognition of the define.xml version (1.0, 2.0)
- Different sets of rules for different define.xml versions
- Only rules developed by the CDISC define.xml team
- Transparent sets of rules ("OpenRules"): the rules are all in user-inspectable, human- as well as machine-readable Schematron files
- No hidden rules (see discussion here)
- File drag-and-drop
- Choice between regulatory and non-regulatory use of the define.xml (different sets of rules!)
- Select / unselect individual Schematron rules
- Reports either as text or RTF files (easily incorporatble into Word documents), no "Excel chaos"
- A really functioning CLI (Command Line Interface) - for a discussion, see here
- Operating System independent (real Java), also deployable on Linux as well as Windows servers, e.g. for batch implementations
- Define.xml v.2.1 ready, no software update necessary, just install additional XML-Schema and Schematron files
- Considerably lower cost than the "Enterprise" version of other validation tools
- Real customer support: questions are usually answered within 24 hours
The Define.xml Checker is used by a many sponsor organizations (big pharma, biotechnolgy companies), CROs, and all major service providers specializing in
generation of SDTM-compliant submissions and legacy data conversion.
Pricing
Single license (perpetual license, PC or server): US$ 1,200 / € 1,000
Free downloads are available for non-profit users: academia, state regulatory organizations, regulatory reviewers
How to order?
The best and easiest way to order is using a purchase order from your organization

XML4Pharma, Katzelbachweg 18, A-8052 Thal, Austria



