
|
||||
| Home | Services | CDISC | Software | About us |
More and more we will find XML-based applications based on the simple scheme given in Fig.1. Data, originating either from human input or from other sources (e.g. measuring equipment) are stored in databases. These data are retrieved by an application using SQL-queries. Then business logic is applied (e.g. calculations). For sofar, there is nothing special about this application.
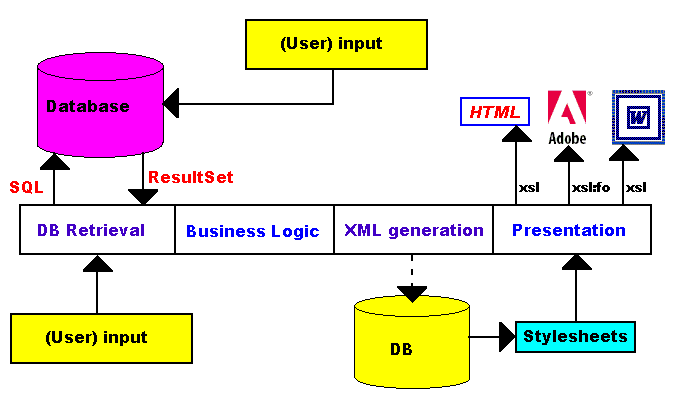
In a web environment, the application will typically be a servlet.
In the next step, the result is transformed into an XML-document, which can
be stored into a database. The XML document is then parsed with one or more
stylesheets, to produce the presentation. For example, using (fully reusable)
different stylesheets, the XML document, is presented as HTML (i.e. a dynamic
web site), a PDF file (using XSL-Format), or even as a Word-document (it can
also be presented as e.g. a WAP-document).
The application is used to produce Analysis Certificates in the Quality
Control lab of a pharmaceutical company (Fig.2).
Data from analytical measurements and human input are stored into a database.
When triggered (e.g. by a human), the application retrieves all the
information about a certain sample (or series of samples) and calculates some
properties, deciding whether the materials conforms, or not, to the
specifications.
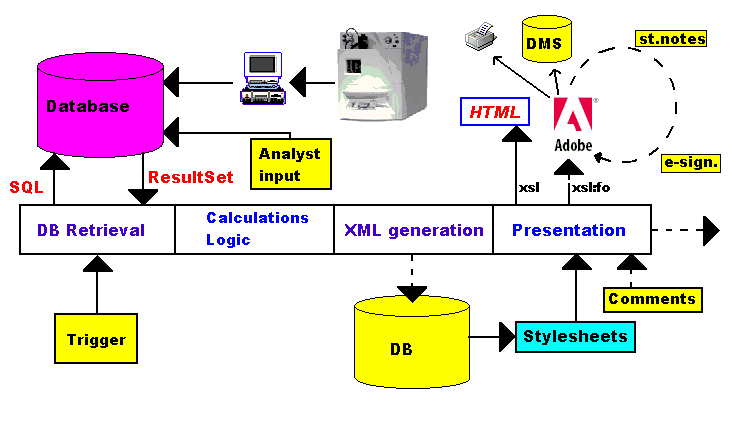
If the specifications are not met, a non-conformity report is generated.
If the specifications are met, an Analysis Certificate is generated. Both are
in XML format, so they contain only data, no presentation. These XML-files
themselves can be stored (and usually are) in a database. Different
stylesheets (XSL) are retrieved from a database or from files, to generate
the presentation of the reports. First of all, an HTML file is generated, so
that the responsible person immediately sees the results, only needing a
browser (on an inexpensive PC or web terminal). Secondly, a PDF document is
generated. The PDF document has the advantage that its layout is fixed,
irrespective of how it is viewed (this is not the case at all with Word
documents), that the contents cannot be changed (important for a
certificate), that "sticky notes" can be added when several persons have to
review the document, and that electronic signatures can be used.
Ultimately, the PDF document can be printed, mailed to the customer, stored
in a database, into a Document Management System such as Documentum, etc...
If the company now decides to give the Analysis Certificate another design (e.g. because the company name changed), all that has to be changed is the stylesheet. It is not necessary to change anything in the application (which would mean an expensive re-validation !).
