
|
||||
| Home | Services | CDISC | Software | About us |
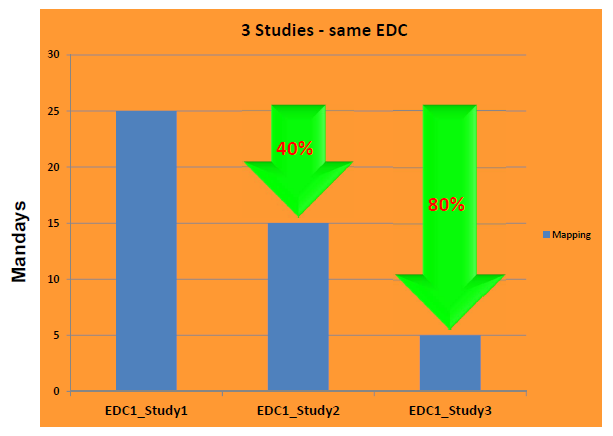 |
Mapping metrics from one of our customersOne of our customers showed this slide during his presentation at the 2015 European CDISC Interchange in Basel. It shows the effort that was needed for generating the complete mapping to SDTM using SDTM-ETLTM, including define.xml (which is kept in sync during the mapping) for three studies using the same EDC system. For the very first study, with still unexperienced mappers, it took an effort of 25 man-days to develop all the mappings, test them, complete the define.xml, and generate all the SDTM files in SAS-XPT format, including supplemental qualifier files, and the CO and RELREC datasets. For the second study, they used the mapping from the first study as a template, and so could reuse a part of the mappings, decreasing the mapping effort to 15 man-days. Having gained experience with the software and with SDTM, the effort for the mapping of the third study further reduced to the incredible low amount of 5 man-days. The reason for this is of course that much of the mappings of the previous studies could be reused, as the data and study design in ODM format came from the same EDC system. |
Need more information? Just drop us an e-mail
