
|
||||
| Home | Services | CDISC | Software | About us |
Many companies currently have started projects to convert legacy data to CDISC standards (SDTM, ODM, Lab).
These legacy data can be in a variety of formats, such as CSV, SAS Transport, XML.
XML4Pharma has the knowledge, software and toolbox available for conversion of legacy data to CDISC standards.
For example, we have developed software to read and write SAS Transport files (without having to need SAS), and to transform XML data to SAS and vice versa.
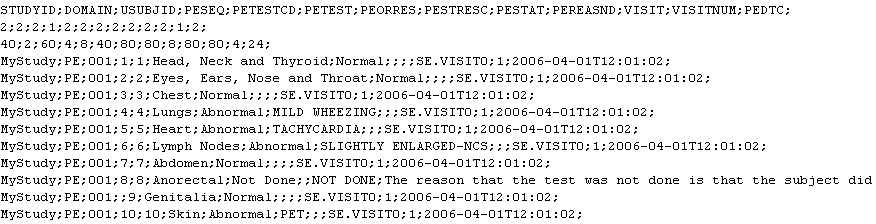
In a typical legacy conversion project, where legacy data need to be converted to SDTM (IG-3.1.1), we take the legacy data (SAS, CSV, Excell, XML -
or a combination of these) and convert them to ODM (or any other XML). The great advantage of using ODM as in intermediate,
is that it generates a full archive in the (by the FDA encouraged) vendor-neutral, open, CDISC ODM standard.
In the second step, the SDTM-ETLTM software is than used to map and transform the legacy data into SDTM.



Other companies do have the SDTM SAS datasets already in place, but do not have the possibility to create the accompanying define.xml file.
If the metadata have been stored in Excell or CSV or XML files (or any other machine-readable format), we can convert these metadata into define.xml (1).
Typically, this is also a two-step process (when the metadata is not in XML): in the first step, the metadata is transformed into simple XML, in the second
step it is fitted into a define.xml file.
Remark: (1) Jozef Aerts of XML4Pharma was one of the co-authors of the define.xml standard, and knows this standard extremely well.
